Stockert 3T System Putting Patients at Risk of Bacterial Infection
In October 2016, the U.S. Centers for Disease Control and Prevention (CDC) issued a warning to doctors and hospitals about contamination risk associated with a commonly-used surgical device. The potentially contaminated Stockert 3T heater-cooler system…
In October 2016, the U.S. Centers for Disease Control and Prevention (CDC) issued a warning to doctors and hospitals about contamination risk associated with a commonly-used surgical device. The potentially contaminated Stockert 3T heater-cooler system is warned to put patients at risk of a bacterial infection known as M. chimaera, which is a form of nontuberculous mycobacterium (NTM).
With around 750,000 patients potentially at risk for being contaminated since the first reports were identified in U.S. hospitals between 2010 and 2014, there is mounting concern about patients being notified of potential exposure. So far, the CDC and the U.S. Food and Drug Administration (FDA) have both received numerous reports of illness and death associated with the NTM bacterial infection after the Stockert 3T system was used.
Read on to learn more about this medical device and the risk of bacterial infection. If you have been diagnosed with a bacterial infection after being exposed to the Stockert 3T system, or another defective medical device, contact the medical malpractice attorneys at MedMalFirm.com. We can help you determine if you are eligible to file a lawsuit and recover damages.
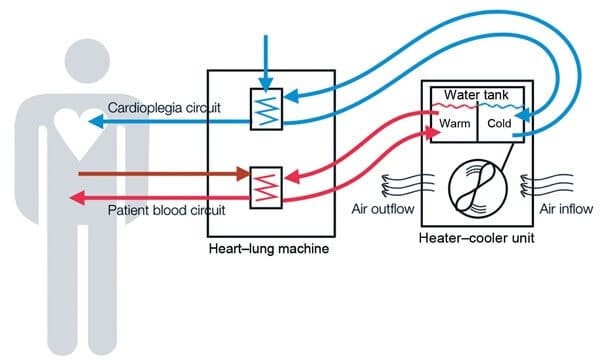
What is the Stockert 3T Heater-Cooler System, and How is it Contaminated?
The Stockert 3T heater-cooler system is a device used to regulate a patient’s body temperature during surgical procedures. Manufactured by LivaNova PLC (formerly Sorin), the device is used in hospitals around the world, primarily during open-heart and cardiac surgeries. During surgery, the device uses a water tank attached to a heart lung machine, oxygenator heat exchanger, or warming/cooling blankets to circulate specific temperature water through circuits that help regulate body temperature. These systems are considered non-sterile, and are not designed to come into direct contact with the patient.
The contamination of the Stockert 3T systems reportedly occurred during manufacturing. In 2014, tests conducted at the facility manufacturing the Stockert 3T systems found M. chimaera bacteria on the production line and in the water supply. LivaNova still processed and distributed the products worldwide. Reports of the contamination did not reach the FDA until October 2015, at which point 32 adverse events reports involving heater-cooler systems indicated NTM bacterial infection.
The water housed inside the tank is reportedly contaminated with bacteria, and then is aerosolized into the sterile operating room environment, including directly into the patient’s surgical site. The particular strain of NTM bacteria associated with the Stockert 3T system is considered by the FDA to be “particularly pathogenic”. This is particularly concerning to the medical community because surgeries using these types of systems occur about 250,000 times each year in the U.S. alone. The number of patients affected worldwide could be staggering.
The risk of contamination, and the lack of warning to hospitals and patients, is also concerning to the legal community. Patients have the right to be informed if they are exposed to a dangerous, defective, or contaminated medical device while under the care of a doctor or hospital. It is unacceptable for patients to suffer harm that could have been avoided had they, or their healthcare providers, been warned of the dangers in a timely manner.
To learn more about your rights as a patient, contact MedMalFirm.com today to speak with one of our skilled medical malpractice attorneys.
CDC Contamination Warning is Not Enough to Protect Patients
In October 2016, the CDC reported that 60-70 percent of bacterial infections from heater-cooler devices were associated with the Stockert 3T system. The FDA started receiving complaints about heater-cooler devices in 2010, with 45 patients across 10 states reporting infection between 2010 and 2016. Of those patients, at least nine died due to serious infection.
The medical community now is stepping up and questioning why more patients were not notified as soon as reports of contamination began. LivaNova discovered the contamination risk in 2014, and the FDA discovered the link between the infections and the Stockert 3T system shortly thereafter, but patients and healthcare providers were not warned about the risk until October 2016.
Hospitals are now taking charge of warning patients, with some U.S. and Canadian hospitals sending out thousands of warnings to patients who may have come in contact with the bacteria. Hospitals and healthcare providers all over the world are stepping up efforts to notify patients and get the word out about the dangers of the Stockert 3T contamination, and the dangers of the M. chimaera bacteria.
There are numerous sources in the media, legal, and healthcare communities expressing concern about the lack of informing patients. One source reported in December 2016 that the FDA did not recommend hospitals inform all potentially exposed patients partially due to the costs involved. This is startling since it is well documented that the best way to treat NTM bacterial infections is early detection and immediate treatment.
What Happens Now to Protect Patients?
With devastating reports being filed all over the world, it is no surprise that lawsuits are also being filed against LivaNova, Sorin, and hospitals using the Stockert 3T system after initial contamination reports were made. Anyone who has undergone surgery using a product like the Stockert 3T and has subsequently been diagnosed with NTM infection should contact their healthcare provider immediately.
Know the Symptoms of Bacterial Infection
Patients should be cautious about any symptoms that are unusual following surgery, and should particularly be aware of the following symptoms associated with NTM infection:
- Fever
- Difficulty breathing
- Heat, redness, or discharge around the surgical site
- Muscle, joint, or abdominal pain
- Nausea or vomiting
- Persistent cough, or coughing up blood
- Weight loss
- Night sweats
Any of these symptoms could be a sign that you have developed a bacterial infection. It is crucial to get medical care as soon as you experience any of these symptoms, as the best way to effectively treat an infection is to start treatment immediately.
Know Your Legal Rights
If you have been injured due to a medical device that was improperly manufactured or used, it is important that you know your legal rights. Manufacturers of healthcare products must be held accountable for their negligence in distributing contaminated products and failing to warn healthcare providers and patients. Get the information and legal support you need by contacting MedMalFirm.com to speak with a skilled medical malpractice attorney. Fill out our online form, and take the first steps toward recovery.
