Essure Birth Control: Benefits, Risks, and Your Rights
If you have ever talked to your doctor about birth control, you probably have heard about a product called Essure birth control. But do you really know what Essure is, or what risks it could…
If you have ever talked to your doctor about birth control, you probably have heard about a product called Essure birth control. But do you really know what Essure is, or what risks it could pose to your health? In this article, our Houston medical malpractice attorney offers a video tutorial about Essure, then explores Essure birth control’s benefits and risks. We will also provide information about how you can get help if you have been injured by Essure or other medical products.
What is Essure Birth Control?
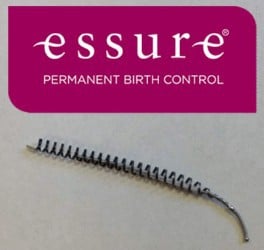
Essure is a non-surgical form of birth control consisting of two polyethylene fibers, stainless steel, and nickel titanium coils. The Essure device is placed the fallopian tubes. After placement, the body reacts to the metal coils by causing scar tissue to form, which blocks the fallopian tubes, thus preventing pregnancy.
Essure is only available via prescription, and is inserted vaginally at your doctor’s office. The Essure birth control device is manufactured by Bayer, which markets it as the only permanent birth control option that is U.S. Food and Drug Administration (FDA) approved, non-hormonal, and can be inserted in a doctor’s office without anesthesia.
Benefits of Essure
The marketed benefits of Essure make it a very tempting option for women looking for permanent birth control. Consider the following marketed benefits:
- When properly placed, Essure is said to be 99.83 percent effective in preventing pregnancy.
- Essure is completely non-hormonal.
- Essure is FDA approved and has been available for 10 years.
- Many insurance companies cover Essure.
- There is little or no downtime after the insertion procedure.
Risks of Essure
Medication errors occur at an alarming rate. Whether it is prescribing the inappropriate medication, improperly inserting a birth control device, or failing to warn patients of the side effects, these errors are dangerous. While it is heralded as one of the “best” and “most effective” methods of birth control, Essure is not without risks. Bayer and Essure warn of the following risks or complications with Essure use:
During Insertion:
- Mild or moderate pain
- Rare cases of breakage of the device, leaving foreign objects in your body
- Rare cases of the device puncturing the fallopian tube, requiring surgery
- Absorption of large amounts of salt water solution into the body
- Complications associated with local anesthesia, if recommended for the procedure
Immediately Following:
- Mild to moderate back or pelvic pain
- Vaginal bleeding
- Nausea or vomiting
- Fainting
- Rare cases of expulsion of the device from the body
- Rare cases of infection
- Essure is not effective until an Essure Confirmation Test confirms that you can rely on it. Until then, you are at risk for becoming pregnant.
- Joint or muscle pain or weakness
- Weight or mood changes
- Persistent fever
Long-term Risks:
- Essure birth control is not 100% guaranteed, and women have reported becoming pregnant while using Essure.
- Women who become pregnant while using Essure are more likely to have ectopic pregnancies (pregnancies developing in the fallopian tubes).
- Women who have NovaSure procedures to lighten or stop menstrual bleeding following insertion of the Essure device may have an increased risk of pregnancy.
- Women who are allergic to nickel may experience a reaction to Essure, including rash, hives, and itching.
- The risk to you and the fetus if you get pregnant after Essure insertion, or via in vitro fertilization (IVF) is unknown.
- Essure can potentially break through the wall of the fallopian tubes and migrate to nearby organs, potentially causing serious injury or death.
Essure Injuries and Legal Matters
Even though the risks of Essure are advertised, it is still often recommended for women as an alternative to medication or surgical birth control methods. Since its release, more than 750,000 women have used Essure. Serious injuries from Essure are marketed as being “rare,” but thousands of women have come forward in recent years claiming they have suffered greatly due to the device. Numerous women have further come forward alleging that clinical trials were tampered with and/or falsified in favor of the manufacturer.
Product manufacturers are required to thoroughly test their products before distributing them. If there are risks, defects, or contamination, they are required to take steps to ensure that consumers are not using a defective product. They are also required to warn healthcare providers of any potential risks so that patients can be informed about risks before agreeing to any procedure or device.
If you have been injured by a medical device, and you believe that you were not adequately informed about the risks or side effects, contact MedMalFirm.com to learn more about your legal rights. Patients should never suffer due to the substandard or inadequate actions of a manufacturer or healthcare provider.
FDA Action
Adverse events reported to the FDA include serious injuries leading to death, such as uterine perforation, air embolism, and infection. The FDA has also reported five fetal deaths caused by Essure. After receiving thousands of adverse events reports overall, the FDA did not ban Essure, but rather, in 2016 did the following:
- A boxed warning — sometimes called a “black box” warning, the FDA’s strongest — and Patient Decision Checklist will now be added to the device’s labeling to help women receive and understand information regarding the benefits and risks of Essure.
- A new, mandatory clinical study has been ordered to determine heightened risks for particular women. (These types of studies are very expensive and can take years to complete.)
- Doctors should provide information and counsel patients on the real risks of Essure birth control.
- Doctors need more training in proper management of Essure patients.
- Doctors should be selective when choosing patients as candidates for Essure.
- Women who have a history of uterine surgery, pelvic pain, or autoimmune disorders should not use Essure.
After these measures were taken, the FDA reported a 70 percent decline in Essure sales.
In 2018, the FDA issued an order restricting the sale and distribution of Essure birth control pending “reasonable assurance” that women are getting adequate information about the device and the possible side effects and risks. The FDA had received numerous reports that women had not been adequately informed prior to the device being implanted.
Can Patients Sue Bayer?
Based on the Medical Device Amendments Act (MDAA), suing a company like Bayer is extremely difficult. Under the MDAA, the FDA has what is called a preemption, which is designed to protect manufacturers from legal liability if their products are found to be faulty or cause injuries. In an effort to stop FDA preemption and allow injured victims the opportunity to fight for justice, a petition has been initiated by none other than Erin Brockovich, a strong advocate for women’s rights.
Thousands of women have come forward with complaints about injuries and side effects related to Essure birth control. There are also documented deaths related to Essure use. It is no wonder, then, that there have been a multitude of lawsuits filed against Conceptus (former manufacturer) and Bayer (current manufacturer). Unfortunately, justice for these women and their families seems difficult to reach.
In April 2016, a California judge moved to allow a number of lawsuits against Bayer concerning Essure to proceed in court. Around the same time, Illinois and Pennsylvania also allowed lawsuits to move forward, and other states considered the same. The lawsuits range in complaint, with many alleging:
- Negligent or fraudulent manufacture
- Negligent training
- Negligent risk management
- Failure to warn of the risks
- Breach of express warranty
Even with so many lawsuits across the country, the Essure lawsuits have not been consolidated or given “class-action” status at this time.
Essure Birth Control and Your Rights
If you have used Essure birth control and have suffered adverse side effects or related health conditions or surgeries, you may find it helpful to contact a medical malpractice attorney to discuss your legal rights. Battling a medical device company like Bayer is challenging, and not all law firms are up to the task. At MedMalFirm.com, we know how complicated these cases can be, and we have the knowledge and experience you need to get results.
If you have questions or concerns about Essure and your health, or your legal rights, contact MedMalFirm.com today. Our team of attorneys is dedicated to protecting your rights and pursuing justice.
